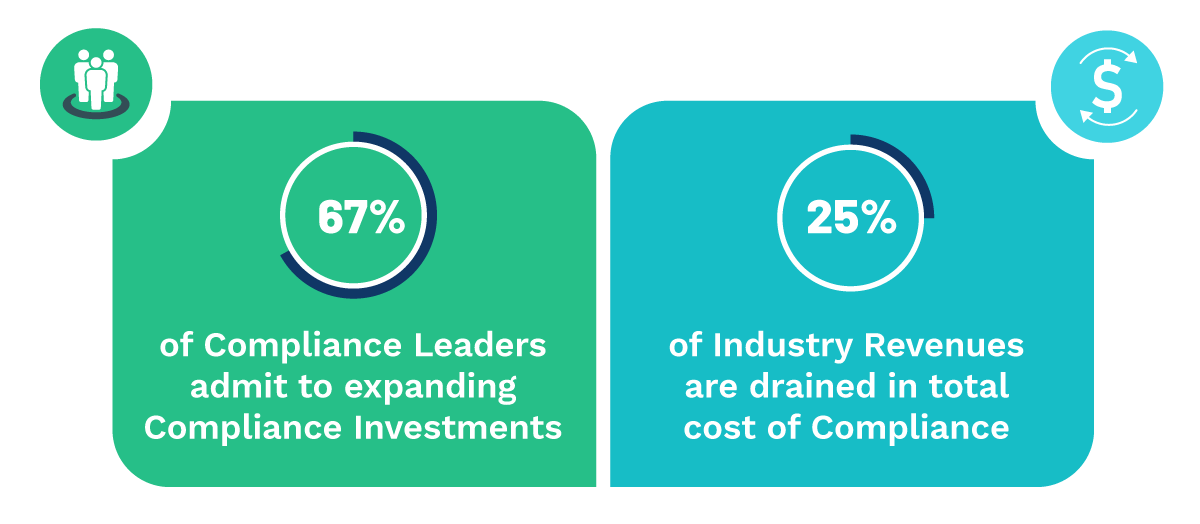
Non-compliance causes soaring secondary costs, including non-released stocks, product recalls, problems with authorities' suspended production, and other risks.
To resolve this, Life Sciences companies must speed up time-to-market, optimize costs, and enhance business models while remaining competitive and complying with a stringent and mercurial regulatory environment.
Compliance Solutions Life Sciences Companies
Jade Global's Managed Services model uses innovative processes and tools to help Life Sciences companies achieve Continuous compliance with their Enterprise Systems.
Life Sciences companies operating in a GXP-regulated environment must maintain ongoing compliance with their Enterprise systems supporting core business processes.
Need for Continuous Compliance Management for Enterprise Systems
Enterprise Systems constantly undergo changes triggered via New Business requirements or periodic Software/Infrastructure updates that must be governed via a robust change management process to ensure compliance. Especially with companies adopting Cloud Applications, updates happen every Quarter, and the changes will need to be regression-tested and Validated to maintain compliance.
Enabling continuous compliance in the United States per FDA 21 CFR Part 11 regulations needs all Changes to the systems to be Verified & Qualified to meet the Computer Systems Validation guidelines. Computer System Validation (CSV) is the documented process of assuring that a computer system does exactly what it is designed to do in a consistent and reproducible manner. All changes to the System are governed via approved SOPs having established processes and controls.
Continuous Compliance Testing
Validation's verification process includes reviewing and approving changes to specifications and Systems. Validation's qualification process is the formal testing of requirements documented in URS, FS, or Design documents. You perform these tests during the Infrastructure Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) stages of the validation process.
Ongoing changes to the Enterprise platforms include planned minor & major releases and unplanned Emergency Fixes. In addition, there are significant project-driven changes, either updating or adding to the existing footprint. The events triggering the change need to be assessed for impact on the Core Business process & any new functionality is enabled so the scope of testing is established and Test execution can be planned.
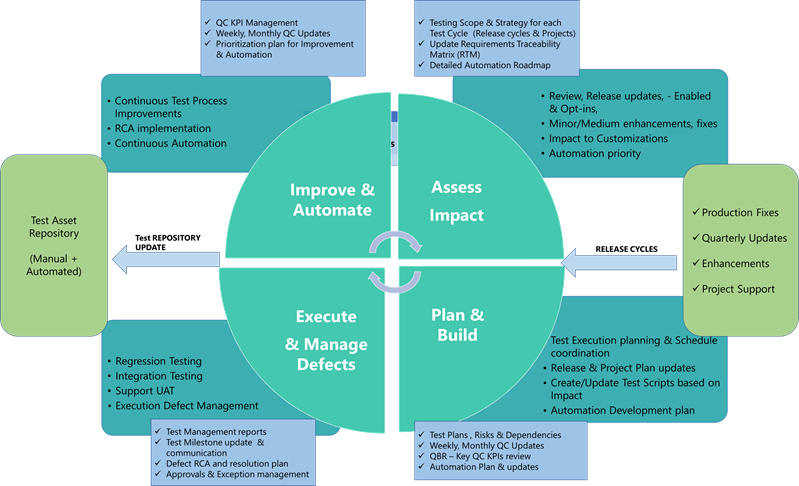
While it is important to accommodate regression testing & formal Validation process to maintain continuous compliance through release cycle changes, it is equally vital not to slow down Time-to-market in deploying changes to production.
How Jade Global's Managed Services seamlessly meet Compliance Objectives:
The absence of an enterprise-wide view of compliance risks is very much prevalent in Life Sciences Companies.
Jade Global has embedded a robust Change control process into Managed Services Methodology, leveraging Industry Standard ITSM processes and Tools for electronically reviewing and approving the Changes. It has integrated Automated Testing-as-a-Service Offering into the Managed services model to help Accelerate and comprehensively run the Regression and Formal Testing process to meet compliance objectives and maintain better system quality.
Jade Global's automated testing framework promotes continuous Automation. It helps with the Velocity of changes deployed to prod with a significant reduction in cost and efforts to perform adequate testing and ensure ongoing compliance.
Conclusion
Essentially change management is a key attribute for validating changes in regulated environments, which puts a lot of overhead on an organization to cater to this.
With Jade Global's effective change management integrated with our managed service offering, we bring the following advantage to customers:
- Template Driven Change Control for Standard Operating procedures.
- Automating Workflow for changes and evidence collection.
- Automating deployment and testing to validate the changes.
A robust managed service operation powered by effective tools reduces overheads for organizations to validate and manage changes in a regulated industry.













