GxP Systems Validation Process for Life Science and Pharmaceutical Industry
When it comes to compliance, life sciences companies are a lot like Goldilocks.
In the famous children’s fable, the young girl sampled porridge and chairs and beds of the three bears before finding the ones that were “just right.”
Life sciences companies can relate as they deal with the challenges of complying with federal regulations that require IT systems to function under the principles of GxP, short for “good practice” in a given (x) field.
Obviously, life sciences companies can’t afford to cut corners on IT systems compliance mandated under 21 CFR Part 11, a section of the U.S. Code of Federal Regulations (CFR) requiring GxP validation of technology implementation, operation, and performance.
But they don’t want to over do it, either. Erring on the side of excess in GxP validation (compliance) can mean additional time, cost, and resources with no benefit to any stakeholder — the life sciences company, regulatory bodies, or healthcare consumers. And it diverts the business from its core focus of developing therapeutics to prevent and alleviate health conditions.
GxP Compliance Pharmaceutical Industry
Rightsizing GxP compliance is the sweet spot, but it can be tricky. It requires deep expertise in 21 CFR Part 11 regulatory requirements governing pharmaceutical, biotech, and medical device firms. And it demands in-depth knowledge of IT systems. Done right, GxP compliance contributes to quality and integrity across product and supply chain lifecycles.
Goldilocks needed just a few minutes to find the “just right” porridge. It could take a life sciences company years of trial and error to develop a “just right” approach to GxP compliance and right partners to provide GxP compliance services.
Challenges and Risks of GxP Systems Validation Process (IT Systems Validation)
Some life sciences firms choose to tackle GxP compliance on their own. They invest heavily in internal teams of compliance and IT professionals to sort out compliance requirements across enterprise IT systems, with reporting to the U.S. Food and Drug Administration (FDA).
That brings high overhead of time and cost, plus the challenge of attracting and retaining personnel that specialize in GxP compliance and IT systems validation — funds that a company would prefer to spend on a molecular biologist, epidemiologist, or clinical technician.
GxP compliance is a complex task that applies to a range of enterprise systems, from ERP applications and cloud platforms like AWS to software for quality control, manufacturing, warehousing, and cloud-based integration such as the Boomi AtomSphere Platform.
Compliance becomes even more difficult as IT environments evolve with new cloud applications, software updates, and changing business requirements. As systems change, GxP compliance documentation needs to be shared with the FDA — and made available on demand in the event of an FDA audit.
As I noted earlier, life sciences firms certainly don’t want to be cutting corners: Noncompliance can bring stiff FDA penalties, potential loss of FDA licenses, product recalls, and unsold inventory. Similar requirements and penalties are in place in countries around the globe.
Sidestepping GxP Compliance Cost and Complexity
At Jade, a Boomi Elite Partner offering IT and business consulting services, we specialize in helping life sciences companies achieve GxP compliance with the Boomi AtomSphere Platform and other enterprise systems. Providing a Complete package of GxP compliance services.
Our main focus is pre-revenue startups and small to midsize life sciences companies with revenue up to $250 million a year, as they often lack the in-house expertise and more mature IT systems seen at larger organizations.
With deep experience in both regulatory compliance and IT systems, our team helps life sciences companies sidestep common business challenges around GxP compliance:
Complicated processes- The burden of necessary processes (e.g., regulatory compliance, quality assurance, change management) stresses the organization with high overhead and incremental workloads that can erode a company’s core focus.
Wide variability in cost and effort-GxP compliance costs should ideally be 5 percent or less of the total implementation cost of a new system. However, costs can rise to 25 or 30 percent without the right team and proven repeatable GxP systems validation process.
Understanding the “just right” balance point between underdoing and overdoing GxP compliance is essential. And going forward, that knowledge supports ongoing compliance change management as IT systems and business requirements evolve.
As life sciences leaders know, GxP is just one of several compliance challenges confronting the industry. In a previous blog post, I outlined how pharmaceutical manufacturers also need to comply with the FDA’s Drug Supply Chain Security Act (DSCSA), designed to combat counterfeit drugs and support recalls.
GxP Compliance for Enterprise iPaaS
So how does GxP validation and compliance work? Let’s take an example of a life sciences company that’s engaged Jade Global to implement the Boomi AtomSphere Platform for integration across internal and external partner systems.
The client can select Jade’s pre-built validation jumpstart kit, including templates and frameworks, to handle GxP validation compliance with an internal team. Or the client can have Jade handle end-to-end validation processes as Boomi is implemented.
In addition, companies can choose ongoing GxP validation change management as a Jade Global managed service. In that scenario, we supply automated industry-standard IT service management (ITSM) processes and tools for regression testing and validation as Boomi cloud updates are released.
As depicted in the diagram below, three primary validation deliverables are designed to ensure that Boomi is 1) installed as specified 2) operates as designed, and 3) performs per specifications.
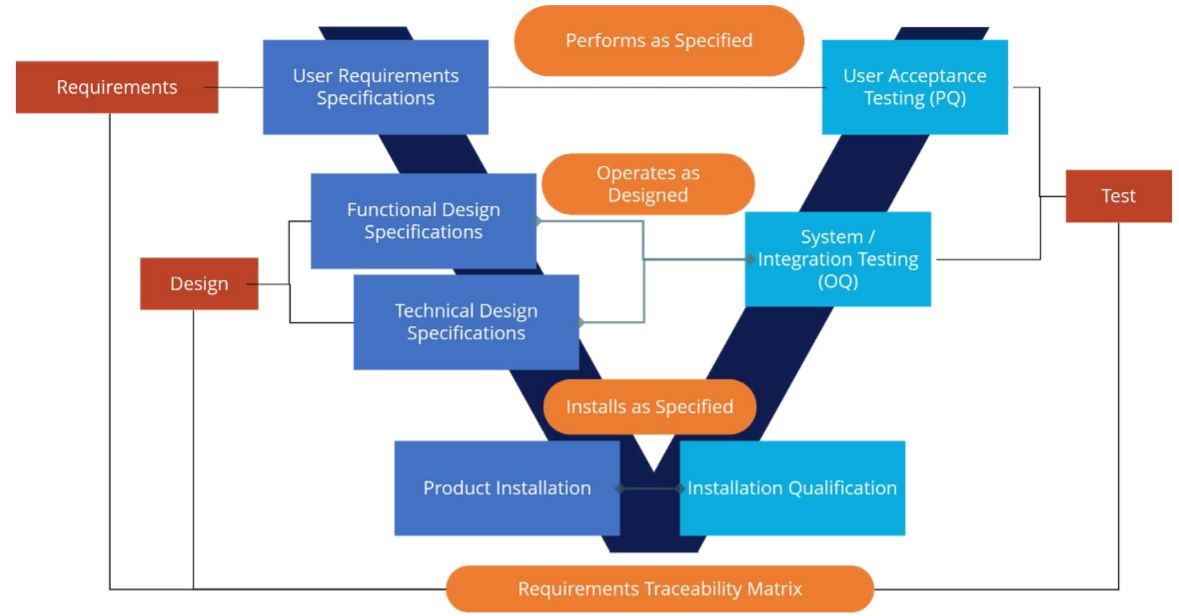
Those deliverables are mapped into a validation methodology based on GAMP 5 (Good Automated Manufacturing Practice Version 5), widely used in life sciences as a risk-based approach to GxP-regulated computerized systems. Some of the steps in validation include development of:
- A comprehensive validation plan
- User requirements and a functional risk assessment
- Functional and technical design specifications
- Protocols and reports for installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ)
- A traceability matrix and validation summary
- Training materials and work instruction
Finally, the life sciences company gains continuous visibility into the status of GxP compliance status. Anomalies that would be difficult to detect manually are highlighted for swift investigation and resolution, eliminating potential obstacles that could disrupt compliance and organizational focus.
A Holistic Approach to Top Life Sciences Objectives
GxP compliance is not a simple undertaking, but it is much more straightforward for organizations that are creating a unified enterprise ecosystem anchored by a best-in-class integration platform as a service (iPaaS).
At Jade Global, the Boomi AtomSphere Platform is our iPaaS of choice to help life sciences firms accelerate cloud-first IT modernization efforts and broader digital transformation. As I noted in an earlier blog post, integration is vital to unifying systems for digital transformation.
A holistic approach equips a life sciences startup or midsize company to orchestrate, under a single umbrella, three focus areas instrumental to short-term success and long-term prosperity:
- Digital transformation with a unified enterprise ecosystem
- DSCSA-mandated track and trace for end-to-end supply chain visibility
- GxP-compliant IT systems that satisfy 21 CFR Part 11 requirements
By aligning those three initiatives, the company gains speed and agility to achieve top-level objectives of faster time to market and improved operational efficiency while unlocking data for analytics and informed decisions.
With heightened attention on life sciences in the wake of COVID-19, we’re already seeing differentiation between companies that labor forward with siloed legacy systems versus those committed to digital transformation in a modern IT environment that meets regulatory requirements. That gap will only widen in the months and years to come.
To learn more, visit Jade Global and view our webinar with Boomi, “Best Practices for Deploying Boomi Enterprise iPaaS for Life Sciences Companies.” And don’t miss our previous blog posts in this three-part blog series for life sciences, on digital transformation and track and trace for supply chain visibility. |













