When it comes to the practice of Computer System Validation (CSV) the stakes could not possibly be higher. This is especially true for life sciences organizations considering how the industry is changing.
Having improved their software and compliance practices, many organizations are now experiencing the negative impacts of inconsistent implementations or unnecessary complexity that hinders their ability to perform with speed, accuracy, and efficacy.
With respect to life sciences, the regulations are constantly changing and so are the business models BK Srinivas, Head of Healthcare & Life Sciences BU
Brief History of CSV
Over the years, the life sciences, pharma industry has evolved into a high technology business. This evolution brought about new challenges in conforming to regulatory guidelines on quality systems. The initial approach focused on validation of individual technical processes or modules, thus creating an array of individual documents. Regulatory agencies worldwide have been struggling with this issue, looking for ways to provide more confidence that these processes will steadily produce a product meeting its pre-defined requirements and value attributes.
The tip of the iceberg is that it’s a practice of ensuring that any software or hardware which meets their purpose complies with their regulatory guidelines. This process helps to ensure that companies can face up to audits and full-scale inspections from the FDA, DEA, Health Canada, and every other medical related body. If a company cannot demonstrate proof of compliance, they cannot sell their products in those jurisdictions.
We provide insights on how to successfully implement systems and technology that meet regulatory guidelines with Jade Global’s unique CSV offerings.
The Cost of Non-compliance
The Food and Drug Administration (FDA) has made a commitment to communicate with industry in an open and transparent way. As part of their commitment, however, FDA has been increasingly using letters as a means to communicate non-compliance.
These letters are essentially sent by FDA to a manufacturer or distributor detailing the regulatory violations found by FDA during an inspection of the facility. In addition to outlining the violations, FDA includes information on how the company can address them.
As per research by the Ponemon Institute, the cost of non-compliance is about 2.71 times ($14.82 million vs. $5.47 million) that of the cost of compliance; such costs include fines, penalties, etc.
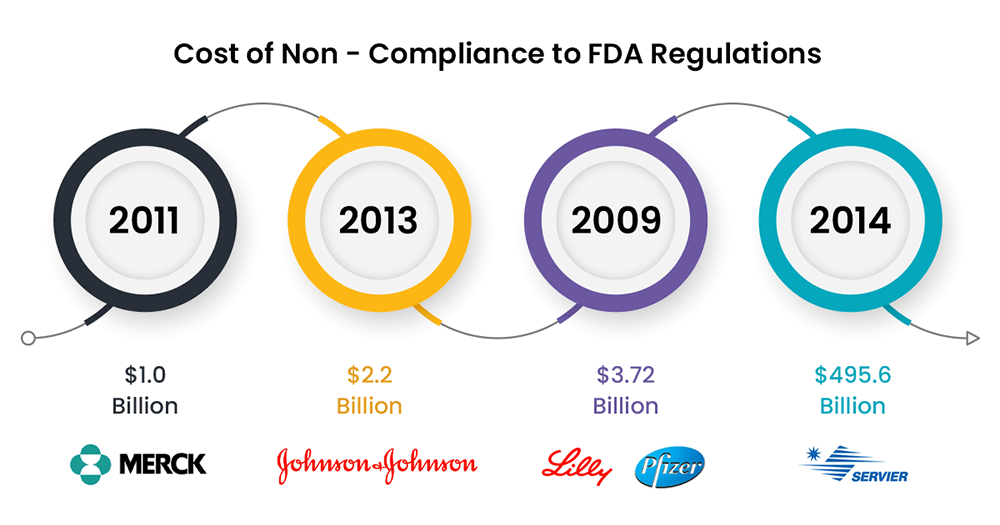
Tens of thousands of products are released worldwide each year, but only a fraction of those win FDA approval. The FDA is constantly updating its testing methods, which can make it difficult for manufacturers to stay compliant. There are certain validations that are deemed germane to compliance like Analytical Method Validation, Process Validation, Equipment Validation, and Cleaning Validation.
How can manufacturers demonstrate that they are keeping pace with these changes? What steps should they take to ensure that they meet all requirements for releasing their products into the marketplace?
Life sciences firms need to
- Demonstrate regulatory compliance in your company's quality systems management plan, validation activities and procedures and manufacturing processes
- Communicate effectively with auditors, inspectors, and other regulators
- Use the best practices for method validation, verification, transfer, and equivalency testing
- Understand the most effective strategies for proving regulatory compliance; and provide successful data from your manufacturing development program
How CSV helps life sciences companies stay ahead of the curve
Life sciences companies produce high-value products that involve numerous complex processes such as: cell line development, biologics manufacturing, formulation, and packaging. All these activities have one thing in common: they require a high degree of quality control. Life Sciences companies are required to demonstrate their compliance with regulatory agencies such as U.S. Food and Drug Administration (FDA). These agencies impose strict requirements when it comes to Quality Control (QC) and Quality Assurance (QA).
Life Sciences companies are required to demonstrate their compliance with regulatory agencies such as U.S. Food and Drug Administration (FDA). These agencies impose strict requirements when it comes to Quality Control (QC) and Quality Assurance (QA).
Reduce risks
CSV runs on each computer or server where regulated data is being handled and provides an audit trail between all phases of production, from design through test, validation, and release phases. This way, you can have evidence that your system works correctly and effectively.
Defect discovery
CSV helps users in the pharmaceutical industry, for example, avoid image losses or fraud. CSV not only helps to protect information integrity, but also enables companies to reduce risk and compliance costs and improve efficiency.
Allows continuous innovation
This system is a necessity for companies that constantly scale and add new features as it allows the development team to prevent tech debt from piling up. It also ensures that new changes don't break existing functionality making sure that every module works in sync with each other.
Another advantage of system validation is continuous improvement by testing and validating every modification before release. This helps in keeping track of all changes for future maintenance requests and planning upgrades or adding new features based on customer feedback.
Reduced operating costs
Production and research organizations have already invested a lot of time and money in their IT infrastructure. The use of validated systems helps reduce operating costs by avoiding re-designs or retrofits in existing systems, which can be very expensive.
Increased labor productivity
Validated software increases labor productivity, as it allows people to spend less time on error correction, improving the overall quality of work by shifting focus from error management to business processes improvement.
Improved business performance
Quality assured computer systems allow businesses to better respond to market opportunities and achieve their strategic goals faster; they enable improved communication with customers and partners; they simplify business processes through automation and provide transparency into critical operations.
Jade Global’s CSV for Enterprise Systems
The need to prepare computer systems for ISO, FDA, and GMP requirements has led Jade Global to create a solution. Our CSV for Enterprise Systems helps life sciences companies from all over the world prepare their computer systems for ensuring regulatory compliance. We take care of your entire validation process, so you can focus your attention and energy on other important business goals.
The roadmap of our engagement
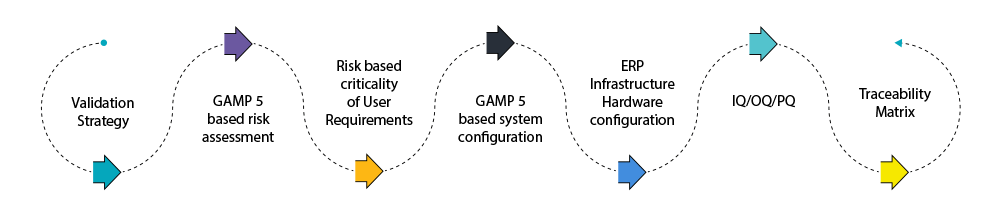
We implement it quickly, minimizing your concerns with data integrity and company growth. It’s quality assurance module provides a flexible set of customizable reports and dashboards, real-time validation alerts, giving you complete control over your compliance. Built upon a proven framework, and dynamic reporting, you can do more with less.
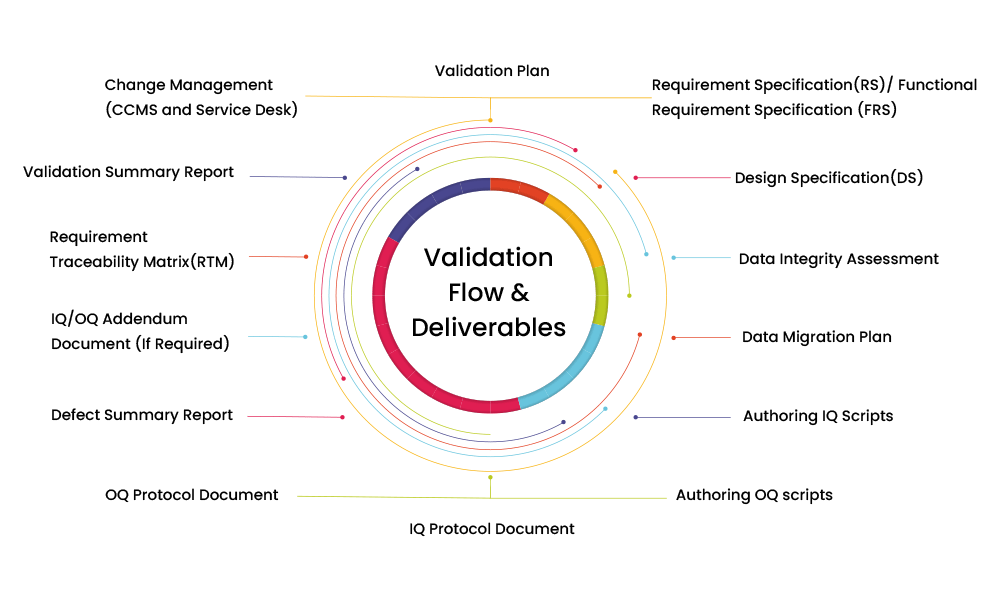
Integration of CSV in QA Testing
- 21 CFR Part 11 guideline has been defined by USFDA regulations to ensure companies maintain good business practices & applies to electronic record and electronic signature of the person working in the regulated environment.
- Enterprise Platforms such as Oracle ERP, SAP and other manufacturing and quality tools needs to be validated and controlled as per GxP guidelines such as 21 CFR Part 11.
- Life Sciences industry can leverage Jade Global’s GxP compliance services and solutions to reduce costs and improve the standard of compliance
- Jade Global can help in applying industry best practices in developing consistent and cost-effective approaches to improve the standard of compliance.
Request for services
Find out more about how we can help your organization navigate its next. Let us know your areas of interest so that we can serve you better.

